Excelerator
The Blueprint Translational Research Group, has developed an innovative model called the Excelerator program to enhance and enable efficient clinical translation through rigorous methods and approaches. This model has supported diverse translational projects and has been funded broadly (e.g., uOttawa Faculty of Medicine, Genome Canada, BioCanRx).
The Excelerator method targets several issues that contribute to failed translation when pre-clinical and early phase clinical trial protocols are developed. Given the broad array of complex issues that prevent efficient bench-to-bedside translation, it is clear that a traditional single-investigator driven approach is simply inadequate to address these challenges. Instead, the Excelerator is a collaborative “team-science” model that leverages various expertise.
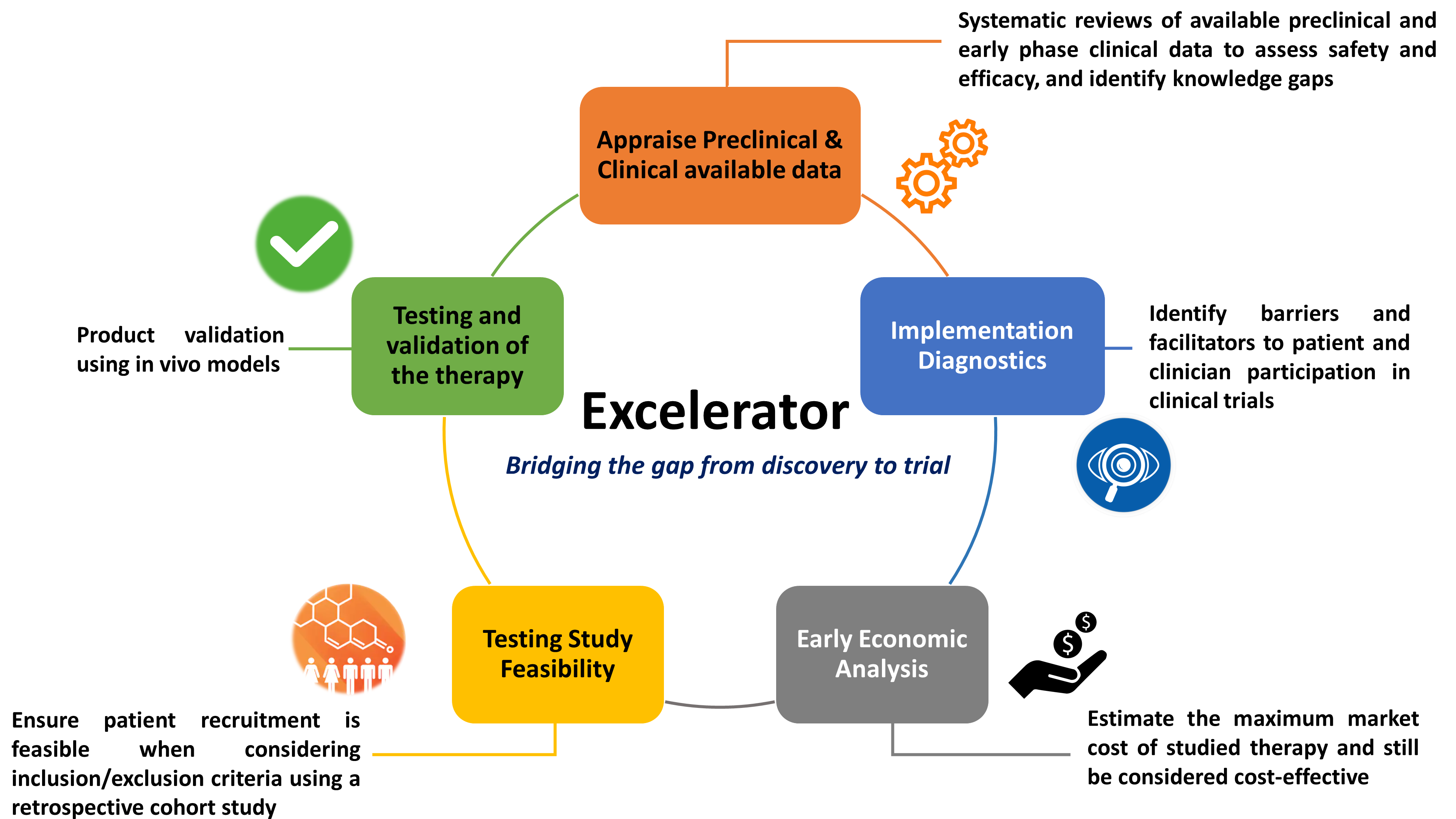
First, we conduct systematic reviews to objectively appraise available preclinical data and early phase clinical data. This allows us to assess safety, efficacy, and identify knowledge gaps. In addition, our reviews have been well received by regulatory agencies (i.e. Health Canada) that are highly concerned about safety when initiating ‘first-in-human’ and early phase clinical trials.
Second, we use ‘implementation diagnostics’ to identify barriers and facilitators to patient and clinician participation in clinical trials. These interview and survey studies (Dr. Justin Presseau) have helped us optimize eligibility criteria, specifics of interventions, and outcomes to be assessed in order to make early phase trials more palatable to these stakeholders. This helps avoid issues with patient recruitment, as 80% of clinical trials fail to accrue sufficient patients in their target time.
Third, we perform early economic evaluation of therapies (Dr. Kednapa Thavorn) to estimate the maximum cost at which they can be brought to market and still be considered cost-effective. Economic implications for novel therapies are often overlooked by investigators, and this delays commercialization and reimbursement processes necessary for clinical adoption.
Fourth, we “test” various planned eligibility criteria using available databases (e.g. The Ottawa Hospital Datawarehouse). These retrospective cohort studies ensure that inclusion/exclusion criteria for an early phase trial remain feasible and a sufficient number of eligible patients could be recruited.
Last, we provide consultation for regulatory requirements, GMP, GLP (Dr. Michael Jamieson, The Ottawa Hospital Biotherapeutics Manufacturing Centre), as well as help with developing clinical trial protocols in order to test and validate the studied therapy.
By addressing many of these issues a priori with a set of focused studies, we can help researchers and clinical investigators delivering optimized clinical trial protocols with the best possible chance of success.
Contacts
Projects that benefited from EXCELERATOR program:
