Labs and Groups
Megeney Lab
Megeney Lab
Team Leader

Lynn Megeney
Senior Scientist, Regenerative MedicineWhat We Do
Our lab studies the molecular mechanisms that control cell adaptation and cell differentiation.
Research Activities
We are particularly interested in the biology of cell death proteins and how these factors are redeployed to promote these beneficial cellular functions. We have focused on the role of the pro-death caspase protease family, a group of enzymes that target and cleave a large numbers of protein substrates. We utilize 2 broad range of model systems to interrogate caspase biology including bakers yeast mammalian cell culture and transgenic animal studies. Our long term goal is to identify the constraints and substrates that allow caspase enzymes to promote stem cell maturation (differentiation) and act as beneficial remodelling agents during cell stress. The work is focused on understanding fundamental biologic mechanisms yet our discoveries may have significant impact for a number of disease conditions including muscular dystrophies, cardiomyopathy/heart failure and tumor initiation/growth.
Caspase control of cell differentiation
Beginning in 2002, we published a series of studies to demonstrate that caspase 3 activation is essential for progenitor cell differentiation, irrespective of cell lineage.We continue to explore this aspect of basic cell biology and are utilizing both proteomic and genomic tools to identify the relevant caspase substrates in this process. We are also exploring whether small molecule activation of caspase 3 can be used as a therapeutic strategy to forcibly engage differentiation and by pass conditions where a block in the differentiation program is a source of human pathology, i.e, Duchenne Muscular Dystrophy and a subset of cancers.

This mural depicts the post natal growth of transgenic mice with the caspase 3 gene removed. Loss of caspase 3 leads to dramatic reduction in body mass, with attenuated skeletal muscle growth (top panels) and disorganized muscle structure (bottom panels). Adapted from Fernando et al .2002, PoD: 12177420
Caspase/metacaspase control of proteostasis
We discovered that the single yeast caspase equivalent (termed a metacaspase) is required for maintaining protein quality control during stress and that loss of this factor leads to the deposition of damaging insoluble proteins. We are continuing to identify the co-factors that assist caspase /metacaspase proteostasis and whether this enzymatic activity can be guided or managed to disperse toxic protein aggregates in a variety of human disease conditions that display loss of prateostasis.
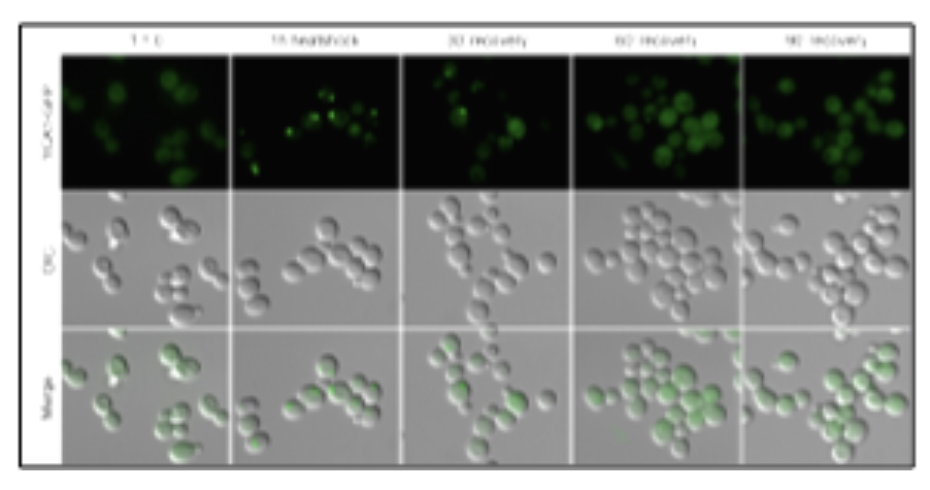
This photo montage displays the subcellular localization of green fluorescent protein tagged yeast metacaspase (yca1)to protein aggregates during heat stress. Adapted from Lee et al. 2010, PMD:20824963
Caspase control of myocardial stress and cardiac hypertrophy
Our observations in the yeast model suggest that caspases may integrate and manage stress responses in mammalian biology, i.e. stress induced growth(hypertrophy) of cardiac muscle. The hypertrophy can be beneficial, as occurs during exercise or pregnancy (physiologic hypertrophy), or detrimental as occurs during pressure overload or with other cardiac disease (pathologic hypertrophy)Our observations suggest that caspase activity is a key rheostat for this growth response and functional proteomic screens are being used to identiy the relevant protein substrates, We are also screening for small molecules and biologist to control caspase activity and direct physiologic hypertrophy, as a therapeutic strategy to counter various forms of cardiomyopathy.
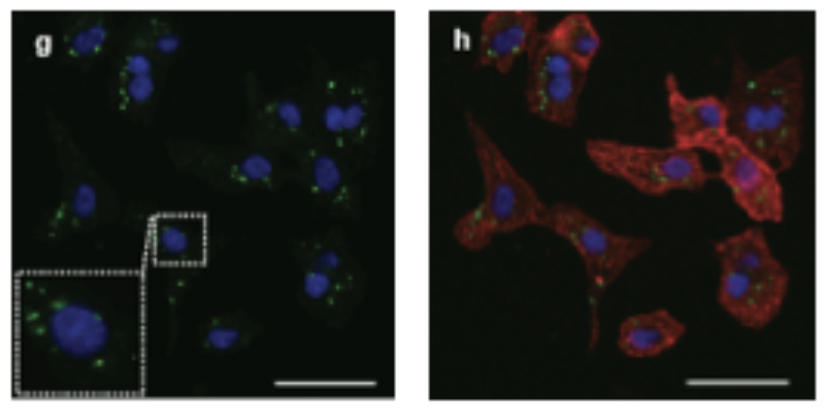
This confocal microscopy image depicts active caspase 3. (green staining) in cardiomyocytes (red staining) during the early stages of hypertrophic growth. The image on the left depicts the active caspase 3{green staining) in close proximity to the cell nucleus (blue staining). Adapted from Putinski et al. 2013,Put 24101
Caspase control of cell differentiation
Beginning in 2002, we published a series of studies to demonstrate that caspase 3 activation is essential for progenitor cell differentiation, irrespective of cell lineage.We continue to explore this aspect of basic cell biology and are utilizing both proteomic and genomic tools to identify the relevant caspase substrates in this process. We are also exploring whether small molecule activation of caspase 3 can be used as a therapeutic strategy to forcibly engage differentiation and by pass conditions where a block in the differentiation program is a source of human pathology, i.e, Duchenne Muscular Dystrophy and a subset of cancers.

This mural depicts the post natal growth of transgenic mice with the caspase 3 gene removed. Loss of caspase 3 leads to dramatic reduction in body mass, with attenuated skeletal muscle growth (top panels) and disorganized muscle structure (bottom panels). Adapted from Fernando et al .2002, PoD: 12177420
Caspase/metacaspase control of proteostasis
We discovered that the single yeast caspase equivalent (termed a metacaspase) is required for maintaining protein quality control during stress and that loss of this factor leads to the deposition of damaging insoluble proteins. We are continuing to identify the co-factors that assist caspase /metacaspase proteostasis and whether this enzymatic activity can be guided or managed to disperse toxic protein aggregates in a variety of human disease conditions that display loss of prateostasis.

This photo montage displays the subcellular localization of green fluorescent protein tagged yeast metacaspase (yca1)to protein aggregates during heat stress. Adapted from Lee et al. 2010, PMD:20824963
Caspase control of myocardial stress and cardiac hypertrophy
Our observations in the yeast model suggest that caspases may integrate and manage stress responses in mammalian biology, i.e. stress induced growth(hypertrophy) of cardiac muscle. The hypertrophy can be beneficial, as occurs during exercise or pregnancy (physiologic hypertrophy), or detrimental as occurs during pressure overload or with other cardiac disease (pathologic hypertrophy)Our observations suggest that caspase activity is a key rheostat for this growth response and functional proteomic screens are being used to identiy the relevant protein substrates, We are also screening for small molecules and biologist to control caspase activity and direct physiologic hypertrophy, as a therapeutic strategy to counter various forms of cardiomyopathy.

This confocal microscopy image depicts active caspase 3. (green staining) in cardiomyocytes (red staining) during the early stages of hypertrophic growth. The image on the left depicts the active caspase 3{green staining) in close proximity to the cell nucleus (blue staining). Adapted from Putinski et al. 2013,Put 24101
Selected Publications
Fernando, P.S., Kelly, J.F., Balazsi, K., Slack, R.S., and Megeney, L.A. (2002). Caspase 3 activity is required for skeletal muscle differentiation. Proc.Natl.Acad.Sci.USA. 99. 11025-11030.
Larsen, B.D., Rampalli, S., Burns, L., Dilworth, F.J. and Megeney, L.A. (2010). Caspase 3/Caspase activated DNase promote cell differentiation by inducing DNA strand breaks. Proc.Nat.Acad.Sci.USA. 107, 4230-4235.
Lee, R.E., Brunette ,S., Puente, L.G. and Megeney, L.A. (2010). Metacaspase Yca1 is required for clearance of insoluble protein aggregates. Proc.Natl.Acad.Sci.USA.107, 13348-13353.
Putinski, C., Abdul-Ghani, M., Stiles, R., Brunette, S., Dick, S.A., Fernando, P.S., and Megeney, L.A. (2013). Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc.Natl.Acad.Sci.USA., 110, E4079-4087.
Dick, S.A. Chang, N.C., Dumont, N.A., Bell, R., Putinski, C., Kawabe, Y., Litchfield, D.W., Rudnicki, M.A. and Megeney, L.A. (2015). Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells.Proc.Natl.Acad.Sci.USA., 112, E5246-5252.
Al-Khalaf, M., Blake, L., Larsen, B.D., Bell, R.A., Brunette, S., Parks, R.J., Rudnicki, M.A., McKinnon, P.J., Dilworth, F.J. and Megeney, L.A. (2016). Temporal activation of XRCC-1 mediated DNA repair is essential for muscle differentiation. Cell Discovery, 2, 15041.
Abdul-Ghani, M., Suen, C., Jiang, B., Deng, Y., Putinski, C., Brunette, S., Weldrick, J., Burgon, P., Fernando, P., Lee, T.T., Flynn, P., Leenen, F., Burgon, P.G., Stewart, D.J. and Megeney, L.A. (2017). Cardiotrophin 1 stimulates beneficial myogenic and vascular remodeling of the heart. Cell Research, 27, 1195-2015.
Shrestha, A., Brunette, S., Stanford, W.L. and Megeney, L.A. (2019). The metacaspase Yca1 maintains proteostasis through multiple interactions with the ubiquitin system. Cell Discovery, 5, 6.
Minina, E.A., Staal, J., Alvarez, V.E., Berges, J.A., Berman-Frank, I., Beyaert, R., Bidle, K.D., Bomancin, F., Casanova, M., Cazzulo, J.J., Choi, C.J., Coll, N.S., Dolinar, M., Fasel, N., Funk, C., Gallois, P., Gevaert, K., Gutierrez-Beltran, E., Halfinger, S., Klemencic, M., Koonin, E.V., Krappmann, D., Linusson, A., Machado, M.F.M., Madeo, F., Megeney, L.A., Moschou, P.N., Mottram, J.C., Nyström, T., Osiewacz, H.D., Overall, C.M., Pandey, K.C., Ruland, J., Salvesen, G.S., Shi, Y., Smertenko, A., Stael, S., Ståhlberg, J., Suárez, M.F., Thome, M., Tuominen, H., Van Breusegem, F., van der Hoorn, R.A.L., Zhivotovsky, B., Lam, E., and Bozhkov, P.V. (2020). Classification and nomenclature of metacaspases and paracaspases: no more confusion with caspases. Molecular Cell, 77, 927-929.
Larsen, B.D., Rampalli, S., Burns, L., Dilworth, F.J. and Megeney, L.A. (2010). Caspase 3/Caspase activated DNase promote cell differentiation by inducing DNA strand breaks. Proc.Nat.Acad.Sci.USA. 107, 4230-4235.
Lee, R.E., Brunette ,S., Puente, L.G. and Megeney, L.A. (2010). Metacaspase Yca1 is required for clearance of insoluble protein aggregates. Proc.Natl.Acad.Sci.USA.107, 13348-13353.
Putinski, C., Abdul-Ghani, M., Stiles, R., Brunette, S., Dick, S.A., Fernando, P.S., and Megeney, L.A. (2013). Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc.Natl.Acad.Sci.USA., 110, E4079-4087.
Dick, S.A. Chang, N.C., Dumont, N.A., Bell, R., Putinski, C., Kawabe, Y., Litchfield, D.W., Rudnicki, M.A. and Megeney, L.A. (2015). Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells.Proc.Natl.Acad.Sci.USA., 112, E5246-5252.
Al-Khalaf, M., Blake, L., Larsen, B.D., Bell, R.A., Brunette, S., Parks, R.J., Rudnicki, M.A., McKinnon, P.J., Dilworth, F.J. and Megeney, L.A. (2016). Temporal activation of XRCC-1 mediated DNA repair is essential for muscle differentiation. Cell Discovery, 2, 15041.
Abdul-Ghani, M., Suen, C., Jiang, B., Deng, Y., Putinski, C., Brunette, S., Weldrick, J., Burgon, P., Fernando, P., Lee, T.T., Flynn, P., Leenen, F., Burgon, P.G., Stewart, D.J. and Megeney, L.A. (2017). Cardiotrophin 1 stimulates beneficial myogenic and vascular remodeling of the heart. Cell Research, 27, 1195-2015.
Shrestha, A., Brunette, S., Stanford, W.L. and Megeney, L.A. (2019). The metacaspase Yca1 maintains proteostasis through multiple interactions with the ubiquitin system. Cell Discovery, 5, 6.
Minina, E.A., Staal, J., Alvarez, V.E., Berges, J.A., Berman-Frank, I., Beyaert, R., Bidle, K.D., Bomancin, F., Casanova, M., Cazzulo, J.J., Choi, C.J., Coll, N.S., Dolinar, M., Fasel, N., Funk, C., Gallois, P., Gevaert, K., Gutierrez-Beltran, E., Halfinger, S., Klemencic, M., Koonin, E.V., Krappmann, D., Linusson, A., Machado, M.F.M., Madeo, F., Megeney, L.A., Moschou, P.N., Mottram, J.C., Nyström, T., Osiewacz, H.D., Overall, C.M., Pandey, K.C., Ruland, J., Salvesen, G.S., Shi, Y., Smertenko, A., Stael, S., Ståhlberg, J., Suárez, M.F., Thome, M., Tuominen, H., Van Breusegem, F., van der Hoorn, R.A.L., Zhivotovsky, B., Lam, E., and Bozhkov, P.V. (2020). Classification and nomenclature of metacaspases and paracaspases: no more confusion with caspases. Molecular Cell, 77, 927-929.