 Co-led by Dr. Manoj Lalu (left) and Dr. Dean Fergusson (right), Blueprint has developed an innovative model called the Excelerator program to enhance and enable efficient clinical translation through rigorous methods and approaches.In 2014, the Lancet published a five-part series about increasing value and reducing waste in biomedical research. It detailed how initially promising findings all too often don’t make it into routine clinical practice nor lead to improvements in healthcare. The series set off a wide-ranging discussion in medical research about how to make the most of the talent and resources we have—and of the discoveries we make.
Co-led by Dr. Manoj Lalu (left) and Dr. Dean Fergusson (right), Blueprint has developed an innovative model called the Excelerator program to enhance and enable efficient clinical translation through rigorous methods and approaches.In 2014, the Lancet published a five-part series about increasing value and reducing waste in biomedical research. It detailed how initially promising findings all too often don’t make it into routine clinical practice nor lead to improvements in healthcare. The series set off a wide-ranging discussion in medical research about how to make the most of the talent and resources we have—and of the discoveries we make.
The Blueprint Translational Research Group was established in 2016 at The Ottawa Hospital, in collaboration with the Ottawa Methods Centre. Co-led by Dr. Dean Fergusson and Dr. Manoj Lalu, Blueprint developed an innovative model called the Excelerator program to enhance and enable efficient clinical translation through rigorous methods and approaches. In 2019, Blueprint partnered with the Faculty of Medicine at uOttawa to extend the Excelerator program to its faculty to help rapidly develop promising therapies from bench to early-phase trials.
“We’re here to support rigorous, evidence-based translation,” says Dr. Dean Fergusson, co-lead on the Excelerator, Director of The Ottawa Hospital’s Clinical Epidemiology Program and professor at uOttawa’s School of Epidemiology and Public Health (SEPH). “That involves taking some of the formative work that’s routine in clinical research and applying it to the early translational phases and, even before that, into confirmatory preclinical work.”
One of these preparatory steps is the systematic review, which combines the disparate existing research and synthesizes the results of many different studies. Whereas individual research papers may disagree, a well-executed systematic review can tease out the trends by affording more powerful studies greater weight and arriving at a more confident conclusion.
Dr. Natasha Kekre, associate scientist at The Ottawa Hospital and assistant professor at the Faculty of Medicine, worked with the Excelerator group to look at what was already known about the risks and benefits of Chimeric Antigen Receptor T-Cell (CAR-T) therapy. After they had analyzed 60 small studies, they found that current forms of this treatment were not overly promising for solid tumors, but appeared much more effective in blood cancers. They also shed light on the kinds of neurotoxicity and inflammation problems that are the main worries around this type of therapy.
Deeply understanding what has already been discovered about a treatment can guide a researcher to formulate the right questions and methods to investigate next. But it’s not as simple designing and launching a study from there. For one thing, the protocols that define which patients to include in your study may set you up to fail.
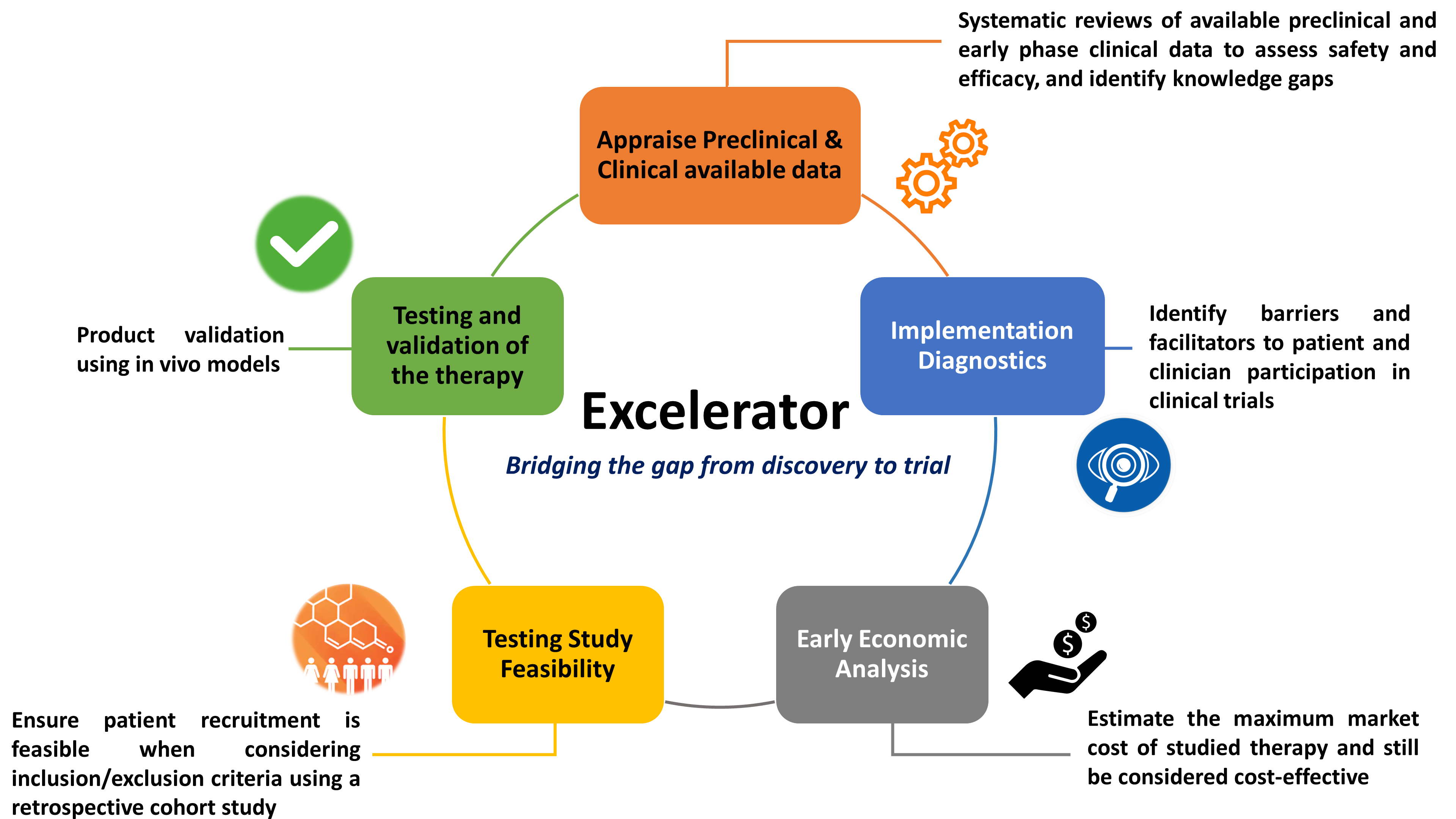
“About 80% of clinical trials have issues with including patients on time. Let’s say a clinical trial plans to include 180 patients within two years. Well, two years rolls around and still they only have 50 patients,” says Mohamad Sobh, a research associate and operational lead for the Excelerator program.
To prevent ending up in that kind of crunch, a researcher can prepare by performing interviews with patient partners and facilitators to determine what barriers exist in recruitment and inform realistic recruitment targets. These studies are led by Dr. Justin Presseau, scientist at The Ottawa Hospital and associate professor at SEPH. Would patients appreciate having the cost of their transportation covered? Does this particular clinician see enough patients with a particular condition coming into their practice to make recruitment feasible?
An approach that involves patients through all stages of a study can bring insights that intuition alone would never have delivered. In 2019, the Excelerator group collaborated on a stakeholder interview study with researchers who were hoping to test the merits of a certain stem cell therapy for chronic stroke.
“What was really interesting there was the patients didn’t seem to care whether it was a safety trial versus an efficacy trial,” says Dr. Manoj Lalu, co-lead on the Excelerator, associate scientist at The Ottawa Hospital and assistant professor in the Department of Anesthesiology and Pain Medicine.. “It shows you how highly motivated these patients might be to participate in an early phase clinical trial. This information also helps inform the design of the planned clinical trial.”
The Excelerator group can also help researchers run a test study. If the plan is to exclude, for example, patients with obesity and those over 65, a retrospective look at The Ottawa Hospital’s data warehouse can reveal how many patients who fit the criteria are likely to walk through the hospital doors within the recruitment window of the study. This type of study helped inform the design of an ongoing CAR-T cell therapy trial.
Finally, health economist Dr. Kednapa Thavorn, senior scientist at The Ottawa Hospital and assistant professor at SEPH, is able to chart out the costs involved in an intervention, set against both the efficacy of the intervention and the comprehensive current costs of today’s care for a patient with this disease. One type of informative analysis she undertakes provides a ceiling price (the maximum price at which an intervention is deemed cost-effective) and, ultimately, a ‘go’ or ‘no-go’ signal before all the expense of clinical trials.
The Excelerator group is now actively looking for research that might be ready, or soon-to-be ready, to take those early steps along the translation pipeline. With the help of the group’s expertise, researchers at The Ottawa Hospital and uOttawa can tackle all the right questions in advance and launch their projects with the best clinical protocol possible.
Learn more about the Fall 2020 Call for Projects, which will provide in-kind support from the Ottawa Hospital Research Institute and the Faculty of Medicine for projects to enter the Blueprint Excelerator program.
For more information about the program, contact Mohamad Sobh at msobh@ohri.ca.
