Research Activities
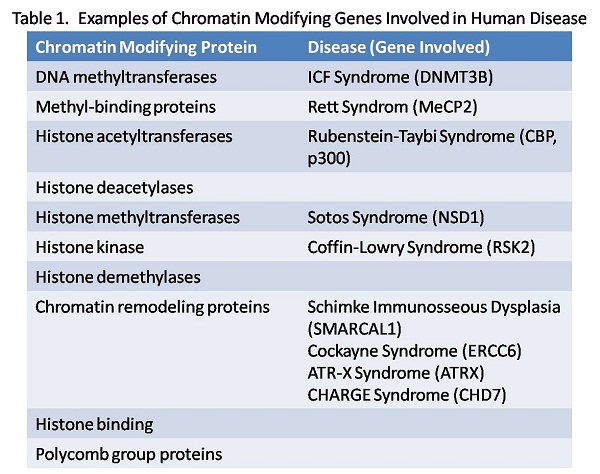
Chromatin remodeling proteins and XLID: Chromatin remodeling proteins play a dynamic role in the regulation of gene expression through the alteration of nucleosome structure (histone acetylation, phosphorylation and methylation) or the ATP dependent repositioning of nucleosomes (SWI/SNF complex, ISWI). Their involvement in genetic disease was established by our cloning of the ATRX gene, a novel SWI/SNF family member that is mutated in a severe X-linked intellectual disability syndrome usually associated with alpha thalassemia. This paradigm has since been extended to include genes encoding almost every type of chromatin modifying protein (see Table 1). We continue to study the role of these proteins in neural development to understand how they contribute to disease pathogenesis. Several current projects are described below.
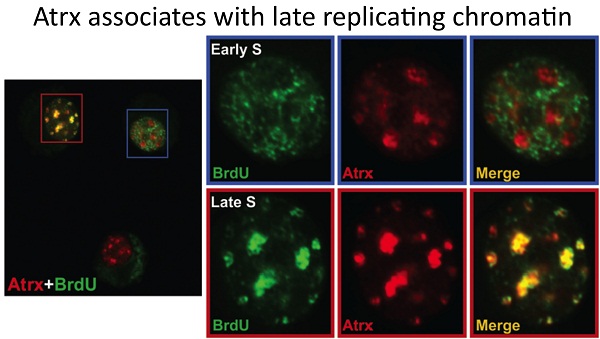
1. ATRX function and the ATR-X syndrome: We make use of multiple Cre drivers to conditionally inactivate Atrx (cKO) in mouse tissues involved in the human syndrome. We have made use of a muscle cKO to define a role for Atrx in replication and/or maintenance of heterochromatin. We identified a cell non-autonomous role for Atrx in the developing retina that affects the survival of inter-neurons. Finally, we are defining the role of Atrx in the developing forebrain. Each of these projects is ongoing and continues to provide fundamental knowledge on the global and cell type specific roles of Atrx.
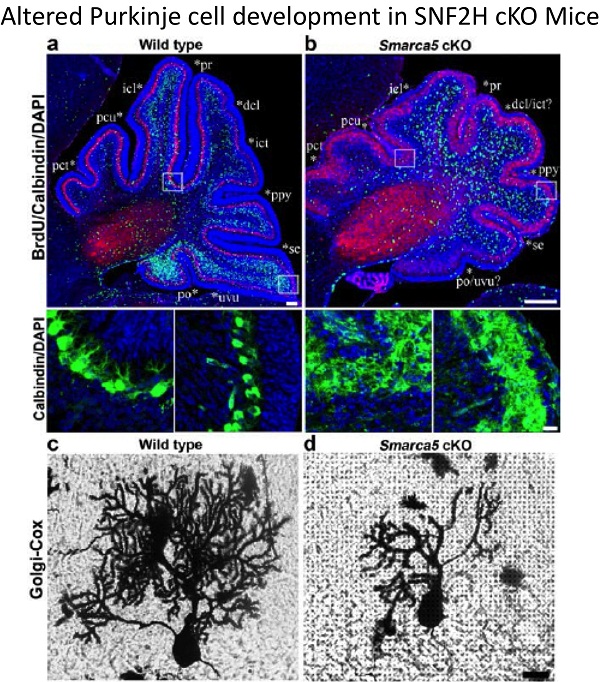
2. Dual functions of the ISWI proteins SNF2H and SNF2L: To extend our analysis of the role of chromatin remodeling proteins in neural development we have characterized the human and murine SNF2H and SNF2L genes. SNF2H is expressed in proliferating neuronal cell populations whereas SNF2L is expressed predominantly in differentiating and/or maturing neurons. Mice inactivated for SNF2L have enlarged brains due to enhanced proliferation whereas mice deficient for SNF2H have proliferative defects and underdeveloped brains. Current lab work aims to understand how these genes regulate common master neuronal developmental homeotic genes to control the size and patterning of specific brain structures.
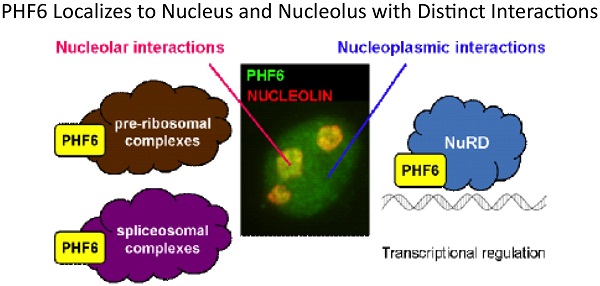
3. PHF6, a causative gene for two distinct diseases: PHF6 contains two PHD-like chromatin domains and is the cause of Borjeson-Forssman-Lehmann syndrome, an XLID, and is also mutated in a significant proportion of T-cell acute lymphocytic leukemia. We undertook a biochemical approach to identify its interacting partners to help elucidate its role in such disparate diseases. We determined that PHF6 interacts with the NuRD complex and future work is aimed at identifying specific targets genes regulated by the PHF6-NuRD complex.