Research Activities
Our research program is focused on understanding the mechanisms which maintain or alter muscle and nervous system integrity and assessing ways to alleviate the pathology of neuromuscular disease, specifically Spinal Muscular Atrophy (SMA) and Multiple Sclerosis (MS). Our research builds on a multidisciplinary approach that includes mouse modeling, genetics, cell biology, and biochemistry.
We are studying the pathogenesis of the genetic disease SMA, which is characterized by progressive degeneration of the large motor neurons in the spinal cord and consequent muscular atrophy. Accumulating evidence, much of it from the Kothary lab, shows that abnormalities in other cell types and organs also contribute to the overall disease burden in SMA. We have generated mouse models of SMA with severe/intermediate phenotypes. These mice are now used in many labs throughout the world. Work from our lab led directly to the use of ROCK inhibitors (which modulate cytoskeletal actin filaments) as a therapeutic for SMA. We have also shown glucose metabolism and pancreatic developmental defects in SMA. This work points to the need for metabolic assessment and therapeutic intervention when considering clinical care management for SMA patients. We have demonstrated intrinsic muscle defects in SMA and most recently described immune organ defects. This latter work raises the question of whether immune dysfunction and neuroinflammation contribute to disease pathogenesis. In other research, we discovered liver abnormalities and lipid metabolic defects in SMA. As therapies for SMA come on the market and children live longer, the expectation is that the extended life span will unmask additional health problems and co-morbidities that will need attention. Collectively, our work highlights SMA as a multi-organ disorder. These unanticipated discoveries of SMA pathogenesis were initially deemed controversial yet are now widely accepted as critical drivers of overall SMA pathology, a discovery initiated and championed by the Kothary laboratory.
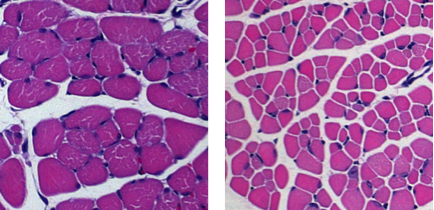 Skeletal muscle atrophy in SMA mice.
Skeletal muscle atrophy in SMA mice.
The other major area of interest is in MS, where immune cells of the
body attack myelin, the protective covering of neurons. Loss of myelin
results in defects in electrical conductance in the neurons and
subsequent problems including difficulties in maintaining balance and
proper gait. Oligodendrocytes are responsible for repairing the damaged
myelin. Unfortunately, in many progressive forms of MS, repair is
attenuated due to the presence of inhibitory factors at the site of
damage. We have developed methods to study oligodendrocyte
differentiation in culture that are now widely used by the field. We are
identifying key inhibitory factors at MS lesions that prevent the full
maturation of oligodendrocytes and halt remyelination in damaged areas
of the CNS. We are investigating the molecular mechanisms underpinning
the failure to repair damage in MS. We have shown that specific
microRNAs are elevated at MS lesions and inhibit the maturation of
oligodendrocytes. We are testing whether acute antagonism of these
microRNAs have therapeutic benefit in a mouse model of MS. The findings
will have an impact on development of future therapeutic strategies
targeting repair of damaged myelin.
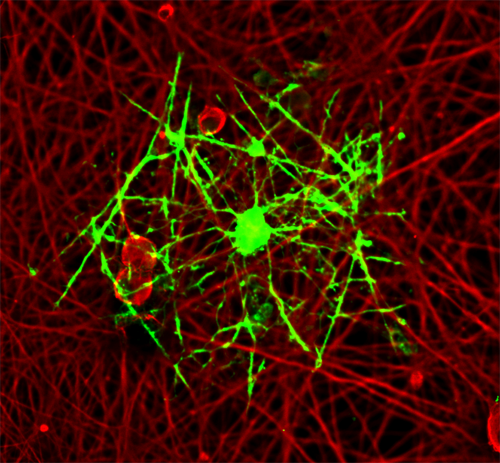 An oligodendrocyte myelinating dorsal root gangion neurite in a co-culture system.
An oligodendrocyte myelinating dorsal root gangion neurite in a co-culture system.
